WHAT IS THE ASTER STUDY?
The ASTER Study is a Phase IV, multi-national, observational, post-launch study to better understand the use of anifrolumab (Saphnelo©) in routine clinical practice among people with Systemic Lupus Erythematosus (SLE).
The key objectives of the ASTER Study are:
- To describe the clinical effectiveness of anifrolumab over time and the duration of effect of anifrolumab in routine clinical practice, as defined by disease activity (Physician Global Assessment [PGA], SLE Disease Activity Index-2000 [SLEDAI-2K]) and the proportion of patients attaining the composite endpoint of Lupus Low Disease Activity State (LLDAS).
- To describe SLE treatment patterns prior to, concomitant with, and after anifrolumab.
- To describe changes in patient reported health-related quality of life, symptoms and impairments.
- To describe healthcare resource utilization for SLE after anifrolumab initiation.
WHAT IS BEING INVESTIGATED?
Anifrolumab is a human monoclonal antibody (IgG1ƙ mAb). It is currently approved in EU, the US, Japan and Canada as an add-on treatment for adult patients with moderate to severe SLE.
Participants in the ASTER Study have been prescribed anifrolumab 300 mg as an intravenous infusion every 4 weeks.
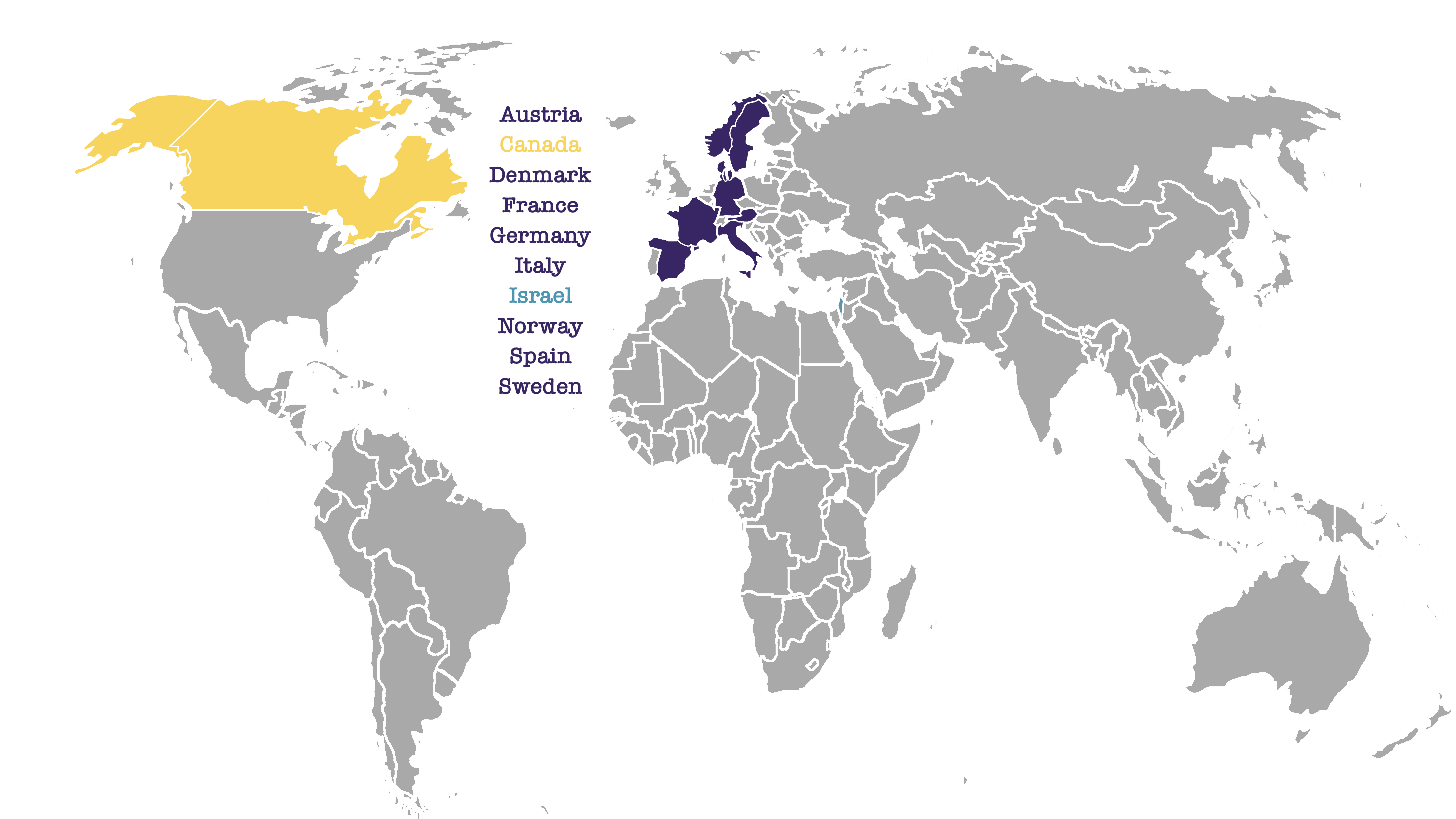
WHO IS PARTICIPATING IN THE ASTER STUDY?
Around 500 patients in 10 countries across the world are expected to take part in the ASTER Study.
You can check the current study status at clinicaltrials.gov
ELIGIBLE PARTICIPANTS MUST:
- Be aged 18 years or older.
- Have fulfilled the 2019 European League Against Rheumatism (EULAR) / American College of Rheumatology (ACR) criteria1 for SLE at the time of study entry.
- Have been prescribed anifrolumab for their SLE treatment for the first time, according to approved country-specific label.
- Have provided their informed consent to participate in the study.
If you think that one of your patients may qualify for the ASTER Study, please contact us for more information. To learn more about AstraZeneca’s commitment to the Lupus community and activities to advance the scientific understanding of Lupus, please visit: https://www.astrazeneca.com/our-therapy-areas/respiratory-and-immunology/lupus.html
References
- 1.Aringer M, Costenbader K, Daikh D, et al. 2019 European League Against Rheumatism/American College of Rheumatology Classification Criteria for Systemic Lupus Erythematosus. Arthritis & Rheumatology. 2019; 71(9):1400−12.
By selecting the button below you acknowledge you are leaving the AZ ASTER Study website and continuing to another website not covered under AstraZeneca’s website Privacy Policy.
Proceed to site